No products in the cart.


If you work in the medical industry, particularly in the pharma industry, you’ve likely heard the terms “primary”, “secondary,” and “tertiary” used in reference to pharmaceutical packaging.
Some medical or pharmaceutical products or medicines may only require primary packaging, some require a combination of primary and secondary, and others require all three.
For the purpose of this blog, we’ll define the common forms of pharmaceutical packaging, before discussing secondary packaging exclusively.
The Different Types of Pharmaceutical Packaging
As discussed, there are three main types of packaging within the pharmaceutical industry. Although this blog focuses on pharmaceutical secondary packaging, in particular, it’s important to understand the purpose of all three and how they work together to protect consumer drugs and pharmaceuticals.
Here’s a little more information about primary and secondary packaging, and tertiary packaging material.
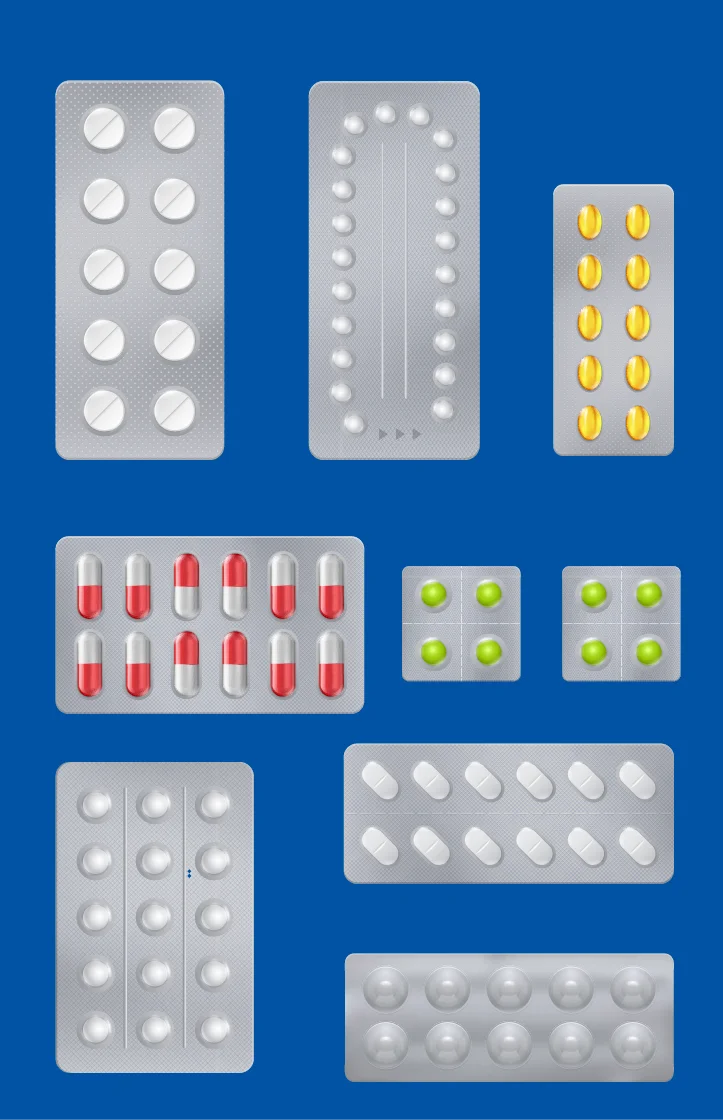

Primary Packaging
Primary packaging refers to any packaging materials that will typically come in direct contact with medication (or the medical device). This includes foil-lid plastic blister packs, a bottle, or single-use packs, which many consumers are already familiar with, either through clinical trials or purchasing medications at the store.
Since this type of pharma packaging comes into direct contact with different types of medication, its main purpose is to protect the medication from extreme heat, extreme cold, and exposure to the elements. It must also achieve a certain level of impact resistance and pliability since it might be dropped on the floor or may fall over on the shelf.


Secondary Packaging
Secondary packaging is the exterior packaging, or overpacking material, that lies outside of the primary packaging. Using the above examples, the manufacturer blister packs (or primary packaging) would be enclosed by a pre printed box or carton at the point of sale -- this box or carton is secondary packaging.
What makes secondary packaging unique is that these packages often include the manufacturer’s branding, a retail message, or dosing information. They’re often brightly colored or otherwise altered to be attractive to patients browsing shelves at the pharmacy. In some cases, secondary packaging may also need to be printed in multiple languages.


Tertiary Packaging
Finally, tertiary packaging is packaging that lies outside of both the primary and secondary packaging. This type of packaging is most often used during delivery and storage (think: big cardboard boxes for delivery, plastic wrap around a case of medication cartons, etc.)
Tertiary packaging is often thrown away shortly after delivery and is not seen by the patient. Compared to secondary packaging, tertiary packaging is bland and plain, as it really doesn’t “matter” what it looks like.
One of the easiest ways to think about these three packaging types and how they work together is that a blister (primary packaging) is placed into a paper carton (secondary packaging) before it is packed into a large cardboard box (tertiary packaging) for shipping. The three different types of packaging work together to protect the medicines during storage, but only one or two are actually visible to the patient.
Essential Elements of Secondary Packaging


When it comes to the main types of pharmaceutical secondary packaging, there are a variety of essential things for a packaging company to consider. First and foremost, it must keep the medication protected from contamination. Not only does this keep the medication free from spoilage, but it ensures potency, which keeps the medication’s effectiveness stable until at least its expiration date. After all, taking medication is pointless if it’s not potent and cannot serve its intended purpose.
Second, secondary packaging should be attractive to the consumer (particularly if the product is available over the counter and competes with all the other products lining the pharmacy’s shelves). It must also accurately and simply describe the product within and provide clear instructions for use.
In some cases, depending on the locale, the instructions included on the pharmaceutical secondary packaging may need to be written in 2-3 different languages. For example, it’s not uncommon for medical products in America to have instructions written in English, Spanish, and Chinese.
Finally, all secondary packaging should adhere to laws and regulations. The medical and pharmaceuticals industries are highly regulated, and many of those rules and regulations apply to packaging specifically.
Secondary Packaging Types & Materials

Though the specific material used depends on the specific use of the secondary packaging, in most cases, secondary packaging is manufactured from the following materials:
Plastics (PE, PP, PET)
LDPE laminates
Aluminum foil
Cardboard
Glass






Plastics are the most popular, though, since many types of medications or medical devices can be safely stored and transported in plastic without sacrificing quality or effectiveness. It’s also very economical and can easily be customized.
Why CarePac
Since our inception, we’ve been designing, manufacturing, and producing secondary packaging for a variety of clients within the pharmaceutical industry. To meet regulatory requirements, we create our pharmaceutical secondary packaging in sterile environments, with only the best line equipment, and all of our products go through strict QA protocols, including serialization.
No matter your needs, CarePac is committed to providing a solution for each and every one of your packaging needs, secondary packaging included. Learn more about our packaging solutions today!
Ready to get started?
Visit our medical packaging page for more information!
Tags

