No products in the cart.
Weed Symbols for Packaging Cannabis, THC, and Marijuana Products

Ah, the ever changing "Universal Symbols" and "universal" warnings for cannabis. It seams that every state has it's own idea of what a universal symbol is, making it hardly "universal" at all. What is evident about the universal symbol, though, is its intention -- to provide warnings and specific statements about cannabis products, so the signs on the product packaging align with state and local cannabis regulations. The universal symbol is used for flower, edibles, and any other consumer cannabis products, whether they're medical cannabis products or recreational.
What is the Universal Cannabis Symbol?
The Marijuana Legalization Act requires the use of an 'universal symbol' indicating that a container, package or product contains cannabis or is a cannabis product.
Each state has its own requirements for what the symbol looks like, but they ultimately all serve one purpose: to signify that the product within contains cannabis.


What does the Universal Cannabis Information Symbol for THC Products Look Like?
We've included a few examples below, and you'll quickly realize that they all look pretty different -- but they also look the same.
Among all the different universal cannabis symbols there are a few similarities: many include marijuana leaves, some include the letters "THC" (which stands for the main psychoactive compound in cannabis), some have explanation marks, and many of them also incorporate a triangle.
Some are more stylistic than others, but they all accomplish the same goal: exclaiming “this is a cannabis product!” to anyone who looks at it.
Why do they all look different?
Since cannabis is not yet legal on a federal level, it is up to each state's cannabis regulation body to come up with their own symbol. For this reason, all the "universal cannabis symbols" look different.
If, or should we say when, cannabis becomes legal on a federal level, we imagine there will be a true universal symbol that replaces all the individual symbols.
Until then, review each state's symbol and required cannabis product information below.
Alabama
Cannabis Regulatory Agency
Alabama Cannabis Labeling Information
Labels, packages, and containers shall not be attractive to minors and may not contain any content that reasonably appears to target children, including toys, cartoon characters, and similar images. Packages should be designed to minimize appeal to children and must contain a label that reads: "Keep out of reach of children."
The following statement shall be included on each label, if space permits, or as an insert within the package: "WARNING: This product may make you drowsy or dizzy. Do not drink alcohol with this product. Use care when operating a vehicle or other machinery. Taking this product with medication may lead to harmful side effects or complications. Consult your physician before taking this product with any medication. Women who are breastfeeding, pregnant, or plan to become pregnant should discuss medical cannabis use with their physicians."
[Ala. Code § 20-2A-63]

Alaska
Cannabis Regulatory agency
Cannabis Regulatory agency
Vector Symbol Download
Additional Alaska Cannabis Labeling Information
Minimum Size: Recommended minimum width of the black triangle is 1/2 inch (12.7 mm) (link)
Required Colors: Contrasting Colors
Required Symbol Background: Dark Background (link)
Placement: On the retail packaging or labeling of every cannabis product
Other Notes: Link here

Arizona
Cannabis Regulatory agency
Additional Info on Arizona Cannabis Information Label
Other Notes: It must contain the following statement: “ARIZONA DEPARTMENT OF HEALTH SERVICES’ WARNING: Marijuana use can be addictive and can impair an individual’s ability to drive a motor vehicle or operate heavy machinery. Marijuana smoke contains carcinogens and can lead to an increased risk for cancer, tachycardia, hypertension, heart attack, and lung infection. KEEP OUT OF REACH OF CHILDREN”.
Click the links before for more information on laws specific to Arizona.

Arkansas
Cannabis Regulatory agency
Additional Arkansas Information
Other Notes: The label includes printable areas for all required information and the warning reads: "Compliant with the State of Arkansas Medical Marijuana Amendment 98. For use by qualified patients only. Keep out of reach of children. Marijuana use during pregnancy or breastfeeding poses potential harms. This product is not approved by the FDA to treat, cure, or prevent any disease."
California
Cannabis Regulatory agency

Mandatory Symbols
Vector Symbol Download

Additional California Cannabis Warning Label Information
Required Size, and Colors for symbol (with citations) | Minimum Size | Required Colors | Required symbol Background | Placement |
|---|---|---|---|---|
Not Available | It must be at least 1/2 inch by 1/2 inch in size | Not Available | Not Available | It must be on the primary panel of all cannabis goods sold in California |
Connecticut
Cannabis Regulatory agency
Additional Connecticut Information:
(a) Prior to delivery or sale at a dispensary, medical cannabis products shall be labeled and in a tamper-evident package. Labels and packages of medical cannabis products shall meet the following requirements:
(1) Medical cannabis packages and labels shall not be made to be attractive to children.
(2) All medical cannabis product labels shall include the following information, prominently displayed and in a clear and legible font:

(A) Manufacture date and source.
(B) The statement "SCHEDULE I CONTROLLED SUBSTANCE."
(C) The statement "KEEP OUT OF REACH OF CHILDREN AND ANIMALS" in bold print.
(D) The statement "FOR MEDICAL USE ONLY."
(E) The statement "THE INTOXICATING EFFECTS OF THIS PRODUCT MAY BE DELAYED BY UP TO TWO HOURS."
(F) The statement "THIS PRODUCT MAY IMPAIR THE ABILITY TO DRIVE OR OPERATE MACHINERY. PLEASE USE EXTREME CAUTION."
(G) For packages containing only dried flower, the net weight of medical cannabis in the package.
(H) A warning if nuts or other known allergens are used.
(I) List of pharmacologically active ingredients, including, but not limited to, tetrahydrocannabinol (THC), cannabidiol (CBD), and other cannabinoid content, the THC and other cannabinoid amount in milligrams per serving, servings per package, and the THC and other cannabinoid amount in milligrams for the package total.
(J) Clear indication, in bold type, that the product contains medical cannabis.
(K) Identification of the source and date of cultivation and manufacture.
(L) Any other requirement set by the bureau.
(M) Information associated with the unique identifier issued by the Department of Food and Agriculture pursuant to Section 11362.777 of the Health and Safety Code.
(b) Only generic food names may be used to describe edible medical cannabis products.
Colorado
Cannabis Regulatory agency
Colorado Marijuana Warning Details
Mandatory Symbols

Vector Symbol Download
In Colorado, the Universal Symbol is required to be marked, stamped, or otherwise imprinted directly onto these products in a distinguishable and recognizable manner:
- Chocolate
- Soft confections
- Hard confections or lozenges
- Consolidated baked goods
- Pressed pills and capsules
When it comes to specific requirements, the symbol should be centered either horizontally or vertically. If centered horizontally, the height and width of the symbol shall be at least 25% of the product’s width and no less than ¼ inch by ¼ inch. If centered vertically, it must be at least 25% of the product’s height and no less than ¼ inch by ¼ inch.
In instances where the product is divided into portions (ex: pieces of chocolate), the symbol must be applied to each portion. In this case, the size of the symbol is determined by the size of the portion instead of the size of the entire product and shall not be less than ¼” by ¼.”
In addition to this universal cannabis information symbol being imprinted directly onto the product, the cannabis packaging itself is also required to include a Universal Symbol marking. Here are the requirements for packaging:

For all types of Medical and Retail

For all types of Medical and Retail
- The Universal Symbol must be located on the front of the Container and no smaller than ½ of an inch by ½ of an inch.
- The following statement must be labeled directly below the Universal Symbol: “Contains Marijuana. For Medical Use Only. Keep out of the reach of children.”
It must also include information about whether or not the container is child-resistant, a complete list of the pesticides, fungicides, herbicides, solvents, and chemicals used, an ingredient list, and must include the following health statements somewhere on the packaging:
- There may be health risks associated with the consumption of this product.”
- “This product contains marijuana and its potency was tested with an allowable plus or minus 15% variance pursuant to 12-43.3- 202(2.5)(a)(I)(E), C.R.S.”
- “This product was produced without regulatory oversight for health, safety, or efficacy.”
- “There may be additional health risks associated with the consumption of this product for women who are pregnant, breastfeeding, or planning on becoming pregnant.”
For full requirements, please view Colorado’s Medical Marijuana Rules.
Delaware
Cannabis Regulatory agency
Additional Delaware Marijuana Warning Packaging Facts
The warning reads: "Medication in this package was produced and distributed in compliance with the Compassionate Use of Medical Marijuana SB 17. It may be legally possessed by a qualified patient. Unlawful to redistribute. Use only as directed by your physician. WARNING! May cause drowsiness. Do not drive or operate heavy equipment while under the influence of this medication. KEEP OUT OF REACH OF CHILDREN."
[Compassionate Use of Medical Marijuana SB 17]
District of Columbia
Cannabis Regulatory agency
District of Columbia Marijuana Warning Details
The label shall contain the following warning:
"There may be health risks associated with the ingestion or use of this product."
Please consult your physician if you have any questions or concerns.” [D.C. Mun. Regs. tit. 22 § C5607]
The warning reads: "Medication in this package was produced and distributed in compliance with the D.C. Marijuana for Medical Treatment Initiative 59 and Bill 18-622. It may be legally possessed by a qualified patient. Unlawful to redistribute. Use only as directed by your physician. WARNING! May cause drowsiness. Do not drive or operate heavy equipment while under the influence of this medication. KEEP OUT OF REACH OF CHILDREN."

Florida
Cannabis Regulatory agency
Mandatory Symbols

Vector Symbol Download
Layered PDF | SVG
Additional Florida Cannabis Warning Details
e. Package the marijuana in compliance with the United States Poison Prevention Packaging Act of 1970, 15 U.S.C. ss. 1471 et seq.
f. Package the marijuana in a receptacle that has a firmly affixed and legible label stating the following information:
(1) The marijuana or low-THC cannabis meets the requirements of sub-subparagraph d.
(II) The name of the medical marijuana treatment center from which the marijuana originates.
(III) The batch number and harvest number from which the marijuana originates and the date dispensed.
(IV) The name of the physician who issued the physician certification.
(V) The name of the patient.
(VI) The product name, if applicable, and dosage form, including concentration of tetrahydrocannabinol and cannabidiol. The product name may not contain wording commonly associated with products marketed by or to children.
(VII) The recommended dose.
(VIII) A warning that it is illegal to transfer medical marijuana to another person.
(IX) A marijuana universal symbol developed by the department.
12. The medical marijuana treatment center shall include in each package a patient package insert with information on the specific product dispensed related to:
a. Clinical pharmacology.
b. Indications and use.
c. Dosage and administration.
d. Dosage forms and strengths.
e. Contraindications.
f. Warnings and precautions.
g. Adverse reactions.
13. In addition to the packaging and labeling requirements specified in subparagraphs 11. and 12., marijuana in a form for smoking must be packaged in a sealed receptacle with a legible and prominent warning to keep away from children and a warning that states marijuana smoke contains carcinogens and may negatively affect health. Such receptacles for marijuana in a form for smoking must be plain, opaque, and white without depictions of the product or images other than the medical marijuana treatment center's department-approved logo and the marijuana universal symbol.
14. The department shall adopt rules to regulate the types, appearance, and labeling of marijuana delivery devices dispensed from a medical marijuana treatment center. The rules must require marijuana delivery devices to have an appearance consistent with medical
use.
15. Each edible shall be individually sealed in plain, opaque wrapping marked only with the marijuana universal symbol. Where practical, each edible shall be marked with the marijuana universal symbol. In addition to the packaging and labeling requirements in subparagraphs 11. and 12., edible receptacles must be plain, opaque, and white without depictions of the product or images other than the medical marijuana treatment center's department-approved logo and the marijuana universal symbol. The receptacle must also include a list of all the edible's ingredients, storage instructions, an expiration date, a legible and prominent warning to keep away from children and pets, and a warning that the edible has not been produced or inspected pursuant to federal food safety laws.
Georgia
Cannabis Regulatory agency
Additional Information
(1) Assign a tracking number to any low THC oil distributed;
(2) Properly package low THC oil in compliance with the federal Poison Prevention Packing Act regarding child resistant packaging and exemptions for packaging for elderly patients and shall label low THC oil with a list of all active ingredients and specific identifying information, including:
(A) The patient's name and date of birth:
(B) The name and date of birth of a caregiver or designated caregiver, if applicable:
(C) The patient's registry identification number from his or her registration card; and
(D) The chemical composition of the low THC oil;
(4) Ensure that the low THC oil distributed contains a maximum of a 60 day supply of the dosage determined for such registered patient; and
(5) Offer access to a licensed Georgia pharmacist to provide professional consultation and counseling, including drug regimen review, for the registered patient.
Hawaii
Cannabis Regulatory agency
Hawaii Cannabis Warning Labeling
The warning reads: "Medication in this package was produced and distributed in compliance with the Medical Marijuana Act 228, SB 862. It may be legally possessed by a qualified patient. Unlawful to redistribute. Use only as directed by your physician. WARNING! May cause drowsiness. Do not drive or operate heavy equipment while under the influence of this medication. KEEP OUT OF REACH OF CHILDREN."
[Medical Marijuana Act 228, SB 862]
Illinois
Cannabis Regulatory agency
Illinois Cannabis warning label
Warnings on labels shall include the following statements:
“This product contains cannabis and is intended for use by adults 21 and over. Its use can impair cognition and may be habit forming. This product should not be used by pregnant or breastfeeding women. It is unlawful to sell or provide this item to any individual, and it may not be transported outside the State of Illinois. It is illegal to operate a motor vehicle while under the influence of cannabis. Possession or use of this product may carry significant legal penalties in some jurisdictions and under federal law."
“Cannabis that may be smoked must contain a statement that "Smoking is hazardous to your health."
“Cannabis-infused products (other than those intended for topical application) must contain a statement "CAUTION: This product contains cannabis, and intoxication following use may be delayed 2 or more hours. This product was produced in a facility that cultivates cannabis, and that may also process common food allergens.”
“Cannabis-infused products intended for topical application must contain a statement "DO NOT EAT" in bold, capital letters.”
Iowa
Cannabis Regulatory agency
Additional Information
A manufacturer shall ensure that all medical cannabidiol packaging is labeled with the following information - A notice with the statement, including capitalization: “This product has not been analyzed or approved by the United States Food and Drug Administration. There is limited information on the side effects of using this product, and there may be associated health risks and medication interactions. This product is not recommended for use by pregnant or breastfeeding women. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.”;
Maine
Cannabis Regulatory agency

Additional Information
Maine’s cannabis labels on prepared marijuana and goods containing marijuana that are sold by dispensaries and caregivers are used as evidence of compliance with the law that limits possession and dispensing to 2.5 ounces of prepared marijuana per qualifying patient. The packaging and labeling of prepared marijuana and marijuana products for sale by registered dispensaries and caregivers must comply with applicable State labeling laws.
Maryland
Cannabis Regulatory agency

Vector Symbol Download
Layered PDF | SVG
Some additional information on Maryland Cannabis warnings.
Minimum Font Size: Any information printed on the package or label must be at least 1/16 of an inch high, which is equivalent to size 4.5 font.
Other Notes: Bear clear warnings that: ○ Products may be lawfully consumed only by the qualifying patient listed on the label.
It is illegal for any person to possess or consume the contents of the package other than the qualifying patient, and it is illegal to transfer the package or its contents to any person other than for a caregiver to transfer to a qualifying patient.
Bear the following statements: “Consumption of medical cannabis may impair your ability to drive a car or operate machinery. Please use extreme caution.”
“There may be health risks associated with cannabis use, especially during pregnancy or breastfeeding.”
“This package contains cannabis. Keep out of the reach of children and animals.”
Massachusetts
Cannabis Regulatory agency
Mandatory Symbols

Vector Symbol Download
Additional Massachusetts Information
Placement: On the retail packaging or labeling of every cannabis product
Michigan
Cannabis Regulatory agency
Cannabis Regulatory Agency
Mandatory Symbols

Vector Symbol Download
Additional Michigan Information
Required symbol Background: The symbol is set on a light grey background to enunciate the white border.

Minnesota
Cannabis Regulatory agency
Additional Minnesota Information
Other Notes: The warning reads: "Medication in this package was produced and distributed in compliance with the Medical Marijuana Laws Chapter 311-S.F.2470. It may be legally possessed by a qualified patient.
Unlawful to redistribute. Use only as directed by your physician. WARNING! May cause drowsiness. Do not drive or operate heavy equipment while under the influence of this medication. KEEP OUT OF REACH OF CHILDREN."[Chapter 311-S.F.2470]
Mississippi
Cannabis Regulatory agency
Department of Health
Additional Information
The following warnings must be highly visible at the point of sale (e.g. at the counter, directly behind the counter at least at eye level):
a. "WARNING: For Medical Use ONLY. Store in a securely locked location away from children."
b. "WARNING: Not for resale. For MEDICAL USE by REGISTERED PATIENTS only."
c. "WARNING: Do not operate a vehicle or machinery under the influence of marijuana."
d. "WARNING: Marijuana should not be used by women who are pregnant or breastfeeding."
e. If products that are intended to be smoked or vaporized are sold: I. "WARNING: Smoking and Vaping is hazardous to your health."
f. If edible products are sold: I. "WARNING: The effects of edible products may be delayed by 2 or more hours".
Label must contain a warning that states "Women should not use marijuana or medical marijuana products during pregnancy because of the risk of birth defects."
Missouri
Cannabis Regulatory agency
Mandatory Symbols

Vector Symbol Download
Layered PDF | SVG
Additional Information
Montana
Cannabis Regulatory agency
Mandatory Symbols

Vector Symbol Download
Layered PDF | SVG

Additional Information
Required Size, and Colors for symbol:
Universal Montana State Marijuana Symbol sized at least .33 inches wide by .33 inches high.
For product packaging, there are some warnings that are required as well:
Must Include:
- “This product has been tested and meets the requirements of the state of Montana”
- “Keep out of reach of children and pets”
- “This product may be addictive”
- “This product may have intoxicating effects. Do not drive under the influence of marijuana.”
May be Required:
- If manufactured: list chemical or solvent used
- If heat is not required to administer or consume: do not include total THC or total potential psychoactive THC on facts panel
Montana Exit Bag Rules
Currently our understanding from speaking with customers is that Exit bags must be Child resistant and contain the following warnings.
WARNING: Consumption of marijuana may cause anxiety, agitation, paranoia, psychosis, and cannabinoid hyperemesis.
WARNING: Consumption of marijuana by pregnant women may result in fetal injury and low birth weight.
WARNING: Consumption of marijuana by nursing mothers may result in infant hyperactivity and poor cognitive function.
Additional Information
Required Colors | Required symbol Background | Placement |
|---|---|---|
Contrasting Colors | Not Available | On the retail packaging or labeling of every cannabis product |
New Hampshire
Cannabis Regulatory agency
Additional Information On New Hampshire
The warning reads: "Medication in this package was produced and distributed in compliance with the New Hampshire Cannabis Program HB 573 (RSA 126-X). It may be legally possessed by a qualified patient. Unlawful to redistribute. Use only as directed by your physician. WARNING! May cause drowsiness. Do not drive or operate heavy equipment while under the influence of this medication. KEEP OUT OF REACH OF CHILDREN."
[New Hampshire Cannabis Program HB 573 (RSA 126-X)]

New Jersey
Cannabis Regulatory agency
Additional Information
Act 241 SLH 2015 - Section 11. Advertising and packaging.
The department shall establish standards regarding the advertising and packaging of marijuana and manufactured marijuana products: provided that the standards, at a minimum, shall require the use of packaging that:
1. Is child-resistant and opaque so that the product cannot be seen from outside the packaging
2. Uses only black lettering on a white background with no pictures or graphics;
3. Is clearly labeled with the phrase "For medical use only":
4. Is clearly labeled with the phrase "Not for resale or transfer to another person";
4. Is clearly labeled with the phrase "Not for resale or transfer to another person";
5. Includes instructions for use and "use by date":
6. Contains information about the contents and potency of the product;
7. Includes the name of the production center where marijuana in the product was produced, including the batch number and date of packaging:
8. Includes a barcode generated by tracking software; and in the case of a manufactured marijuana product, a listing of the equivalent physical weight of the marijuana used to manufacture the amount of the product that is within the packaging, pursuant to section.
New Mexico
Cannabis Regulatory agency
Cannabis Regulatory Commission
Mandatory Symbols
Vector Symbol Download
Layered PDF | SVG

Additional Information
C. Packaging and labeling: a manufacturer applicant shall submit a description and sample of the opaque, child-resistant packaging of the concentrate or cannabis-derived product that the manufacturer shall utilize, including a label that shall contain:
(1) the name of the entity that produced the cannabis and the name of the manufacturer;
(2) a batch number or code;
(3) a production date or expiration date, including a "use by" or "freeze by" date for products capable of supporting the growth of infectious, toxigenic, or spoilage microorganisms;
(4) a description of the number of units of usable cannabis contained within the product;
(5) instructions for use;
(6) warnings for use;
(7) instructions for appropriate storage:
(8) approved laboratory analysis, including the results of strength and composition within ten percent (10%) of numbers shown on the package:
(9) the name of the strain, product facts, or a nutrition fact panel, and a statement that the product is for medical use by qualified patients, to be kept away from children, and not for resale; and
(10) the name of the department-approved testing facility or facilities used for ingredient testing, and the type(s) of testing conducted.
New York
Cannabis Regulatory agency
Mandatory Symbols

Vector Symbol Download
Layered PDF | SVG

Additional New York Cannabis Warning Information
Minimum Size | Required Colors | Required symbol Background | Symbol Placement |
|---|---|---|---|
Minimum size of 1.25 inch in height for the square symbol, 0.5 inch in width for the vertical symbol, and 0.5 inch in height for the horizontal symbol | Required Colors | Not Available | On the upper left 25% of the marketing layer |
North Dakota
Cannabis Regulatory agency
Additional Information
The Packaging must contain Consumer warnings that state:
(a) "This product is not approved by the Food and Drug Administration to treat, cure, or prevent any disease."
(b) "For use by North Dakota registered qualifying patients only."
(c) "Keep out of reach of children."
(d) "It is illegal to drive or to be in actual physical control of a motor vehicle while under the influence of marijuana."

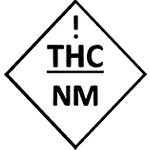
Oklahoma
Cannabis Regulatory agency
Mandatory Symbols

Vector Symbol Download

Additional Information
Minimum Size | Required Colors | Required symbol Background | Symbol Placement |
|---|---|---|---|
No smaller than .6"x.85" in size. | The universal symbol must be red in color; text must be black in color. | Not Available | Not Available |
Pennsylvania
Cannabis Regulatory agency
Additional Warning Information
The label warning reads: "Medication in this package was produced and distributed in compliance with the PA Medical Marijuana Act 16. It may be legally possessed by a qualified patient. Unlawful to redistribute. Use only as directed by your physician. WARNING! May cause drowsiness. Do not drive or operate heavy equipment while under the influence of this medication. "

Rhode Island
Cannabis Regulatory agency
Utah
Cannabis Regulatory agency
Additional Information
Each cannabis and cannabis product label shall contain the following warning: "WARNING: Cannabis has intoxicating effects and may be addictive. Do not operate a vehicle or machinery under its influence. KEEP OUT OF REACH OF CHILDREN. This product is for medical use only. Use only as directed by a recommending medical provider."[Utah Admin. Code R68-28-12]
Vermont
Cannabis Regulatory agency
Mandatory Symbols
Vector Symbol Download
Layered PDF | SVG
Additional Information
Packaging should include the following statement, including capitalization, in at least ten-point Times New Roman, Helvetica or Arial font: ““KEEP OUT OF REACH OF CHILDREN.”
Cannabis products that contain multiple servings, the following statement must be printed on the exterior of the package in a font that is no smaller than ten-point Times New Roman, Helvetica or Arial, including capitalization: “INCLUDES MULTIPLE SERVINGS.”
In the meeting of Cannabis Control Board it was discussed that International Intoxicating Cannabis Product Symbol with or without VT text should be used in the packaging.
Virginia
Cannabis Regulatory agency
§§ 54.1-3442.6 and 54.1-3447 of the Code of Virginia.
18VAC110-60-290. Labeling of batch of cannabidiol oil or THC-A oil products.
A. Cannabidiol oil or THC-A oil produced as a batch shall not be adulterated.
B. Cannabidiol oil or THC-A oil produced as a batch shall be:
1. Processed, packaged, and labeled according to the U.S. Food and Drug Administration's Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements, 21 CFR Part 111; and
2. Labeled with:
a. The name and address of the pharmaceutical processor;
b. The brand name of the cannabidiol oil or THC-A oil product that was registered with the board pursuant to 18VAC110-20-285;
c. A unique serial number that matches the product with the pharmaceutical processor batch and lot number so as to facilitate any warnings or recalls the board or pharmaceutical processor deem appropriate;
d. The date of testing and packaging:
e. The expiration date;
f. The quantity of cannabidiol oil or THC-A oil contained in the batch;
g. A terpenes profile and a list of all active ingredients, including:
(1) Tetrahydrocannabinol (THC);
(2) Tetrahydrocannabinol acid (THC-A);
(3) Cannabidiol (CBD):
(4) Cannabidiolic acid (CBDA); and
(5) Any other active ingredient that constitutes at least 1.0% of the batch used in the product; and
h. A pass or fail rating based on the laboratory's microbiological, mycotoxins, and heavy metals and pesticide
West Virginia
Cannabis Regulatory agency
Additional Information
Any product containing more than 0.3% of tetrahydrocannabinols, or more than 0.3421% of tetrahydrocannabinolic acid, must declare "NOT INTENDED FOR SALE TO PERSONS UNDER THE AGE OF 18" on the label.7.15. Any product label claiming "THC-free" or "non-THC" shall not contain levels of THC above detectable levels as determined by the Department.
[W. Va. Code R. § 61-30-7]
Disclaimer
The contents of this website are intended to convey general information and entertainment purposes only. The information herein should not be relied upon for legal advice on state packaging or marijuana laws. The reader should always consult an attorney in their jurisdiction before making any packaging decisions. The information in this article was not vetted for accuracy by an attorney. We provide links to state cannabis regulatory sites; we do not vouch for or assume any responsibility for the content, accuracy or completeness of material presented in linked sites. The information presented on these pages may not reflect the most current legal developments in the marijuana industry. We disclaim all liability in respect to actions taken or not taken based on any or all of the contents of this site to the fullest extent permitted by law.






